We are pleased to share our publication (McGurk et al. 2024) in Circulation on the pharmacogenetic influences over mavacamten pharmacokinetics.
Mavacamten is a first-in-class, orally administered, cardiac-specific, small-molecule allosteric modulator of β-cardiac myosin. Mavacamten reversibly inhibits the binding of β-cardiac myosin to actin to reduce hypercontractility in an exposure-dependent manner.
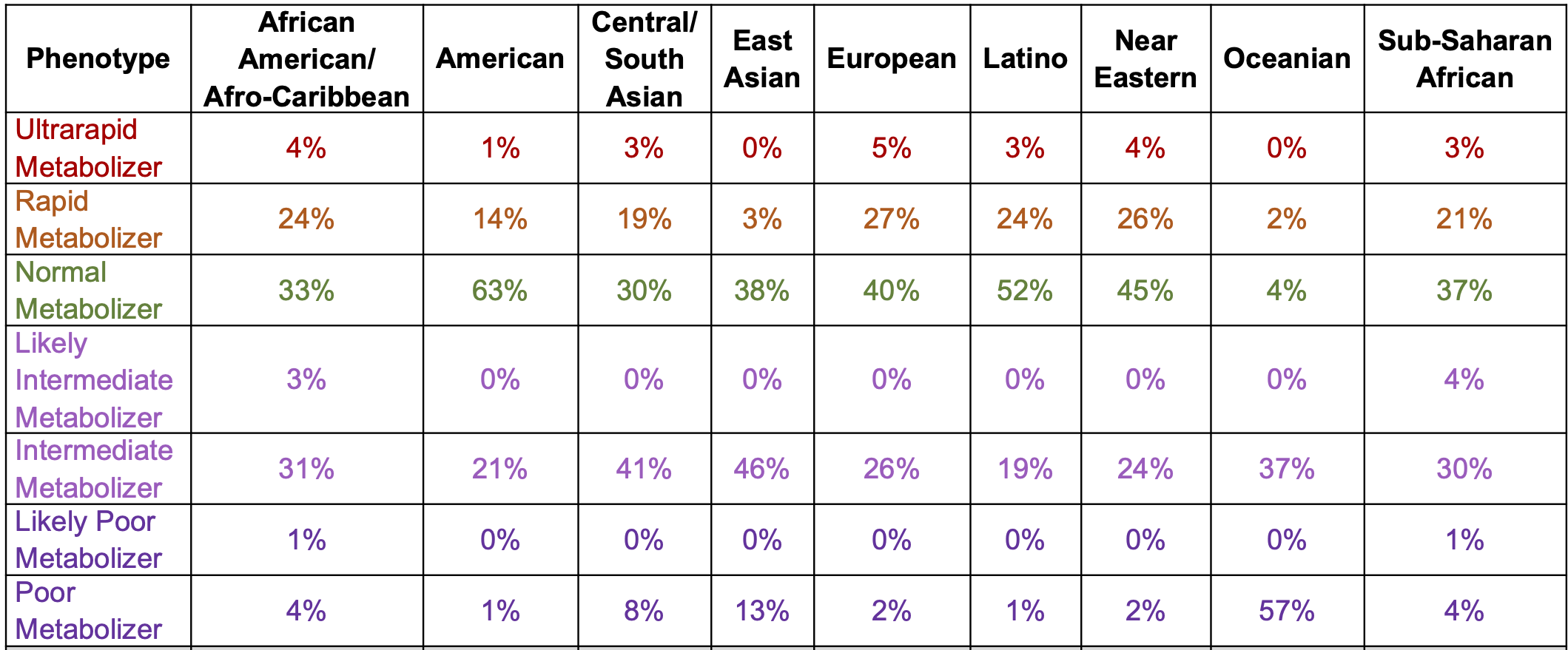
DNA variants can alter the therapeutic and adverse effects of drugs at recommended dosages. Variants in genes encoding cytochrome P450 enzymes, expressed in the liver and small intestine, are associated with variable drug metabolism. Individuals can be categorized into 5 metabolizer phenotypes: poor, intermediate, extensive (sometimes described as normal), rapid, and ultrarapid. CYP2C19 is the dominant metabolizer of mavacamten and poor metabolizers, attributable to diplotypes of 2 CYP2C19 alleles [the CYP2C19*2 (p.Pro227Pro;c.681G>A; rs4244285) and CYP2C19*3 (p.Trp212Ter;c.636G>A; rs4986893) alleles], are at increased risk of systolic dysfunction from mavacamten treatment at the recommended dose. Individual metabolizer status is further complicated by additional variation across other common alleles within this gene, other CYP450 genes, and other relevant pathways.
The prevalence of poor metabolizers (PMs) is common and varies by population (below). The half-life is extended to 23 days in PMs from 9 days in normal metabolisers (NMs). In NMs, it takes ~4 weeks (five half-lives) to eliminate mavacamten from the body after treatment discontinuation.
The EMA and UK Medicines and Healthcare Products Regulatory Agency recommend genotyping for CYP2C19 to determine the appropriate dose. If treatment is initiated without metabolizer status determination, dosage should follow as described for poor metabolizers (starting at 2.5 mg once daily and a maximum dose of 5 mg once daily). The recommended starting dose for all other metabolizers is 5 mg once daily and a maximum dose of 15 mg once daily. Dose modifications are provided for concomitant medicinal products, including CYP2C19 and CYP3A4 inhibitors and inducers. The EMA and Australian Therapeutic Goods Administration suggest a simulated 5 mg dose in a poor metabolizer is similar to the maximum dose (15 mg) in a normal metabolizer.
It remains unclear how the EMA genotyping recommendation will be implemented across diverse European health care systems. Genetic testing for variants in sarcomere-encoding genes is used for individuals with HCM to establish the molecular etiology; pharmacogenetic analysis could be incorporated for individuals who have not already undergone testing and allow for the EMA recommended dosage stratification.
The NICE guidelines note uncertainty surrounding the impact of sarcomeric variants on treatment effect (section 3.7): the relationship between treatment response and genotype has not been fully characterised, with the possibility of differences between individuals with and without sarcomere variants and/or with variants in thick vs thin filament genes.
Providing the appropriate mavacamten dosage to each individual with oHCM from treatment initiation should allow for improved quality of life at the lowest risk of adverse events, cost, and burden to health care systems. Prescribers must be aware of the potential for metabolic variability across and within different ancestries and clinical vigilance with close monitoring will be required to avoid adverse events. Successful treatment requires improving symptoms of oHCM in rapid metabolizers and minimizing the risk of drug-induced systolic dysfunction in poor metabolizers. Treatment without genotyping risks reduced ejection fraction in poor metabolizers or increased time to therapeutic dose in normal metabolizers. With limited medications for the management of oHCM, or where there is limited access to septal reduction therapy, effective titration of cardiac myosin inhibitors is vital to the success of treatment. Whereas future clinical trials with improved metabolizer and ancestral representation will aid our understanding in this area, CYP2C19 genotyping may allow for less frequent clinical monitoring and reduced costs.
We also discuss i) only NICE recommends mavacamten as an add-on to standard care and notes long waiting times for echo; ii) Tian et al 2023 which studied 7 PMs; iii) the potential for predose mavacamten plasma concentration measurement (based on 21 individuals of phase 1 trial).
The NHS England National Genomics Education Programme has also released some guidance and an example clinical scenario
This is just one example of how common DNA variants influence cardiovascular treatment. Pharmacogenetic influences are known and reported for drugs during trials, but to date are used clinically only in a few areas. Further implementation in a cardiovascular setting would allow for reduced adverse events, time to therapeutic dose, and titration, of drug interventions.