Dr Amrit Lota
@AmritLota
We are pleased to share our latest publication in Circulation on the genetic architecture of acute myocarditis and the overlap with inherited cardiomyopathy.
Myocarditis refers to inflammation of the heart muscle. It is traditionally considered to be a random event, which may be triggered by various infections, autoimmune conditions or toxic insults. Whilst most patients show spontaneous recovery, a small but important subset can suffer with long-term adverse cardiac events, including the need for heart transplantation or cardiac device implantation (1).
The purpose of this study was to answer a key question – is there an underlying genetic susceptibility that may explain the wide heterogeneity in clinical outcomes seen with acute myocarditis.
To address this, we compared 3 main cohorts:
– Cohort 1: 230 patients recruited consecutively in London (UK) presenting with acute myocarditis confirmed on cardiovascular magnetic resonance (CMR) or myocardial biopsy (2).
– Cohort 2: 1053 community based healthy volunteers in London with no history of cardiovascular disease and a normal CMR scan.
– Cohort 3: 106 patients presenting with acute myocarditis confirmed on myocardial biopsy in Maastricht (Netherlands).
All participants underwent targeted DNA sequencing for well-characterized cardiomyopathy-associated genes with comparison to healthy controls sequenced on the same platform. The primary outcome was all-cause mortality.
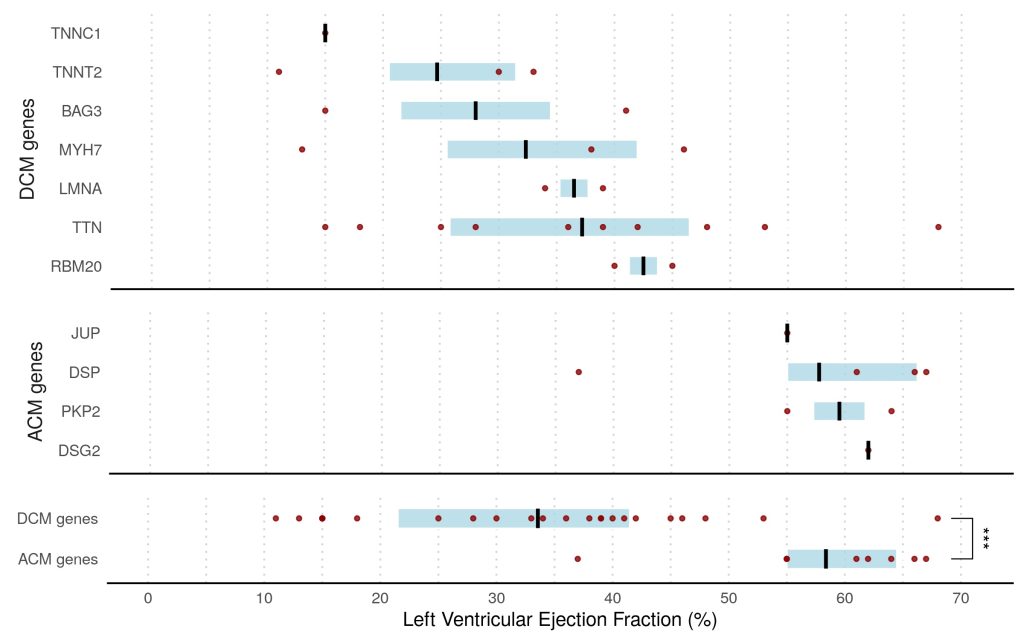
Overall, we found that 8% of patients presenting with acute myocarditis (27 out of 336 cases) carried likely pathogenic variants in ACM or DCM associated genes compared to <1% in healthy volunteers (p<0.0001).
This finding was dominated by truncating variants in Titin (TTN) in 7% of patients, all with left ventricular ejection fraction <50%, compared with 1% in controls (odds ratio, 3.6; P=0.0116). ACM-associated genes were found in 3% of cases versus 0.4% of controls (odds ratio, 8.2; P=0.001). This was driven predominantly by truncating variants in desmoplakin (DSP) in patients presenting with chest pain and preserved LV ejection fraction.
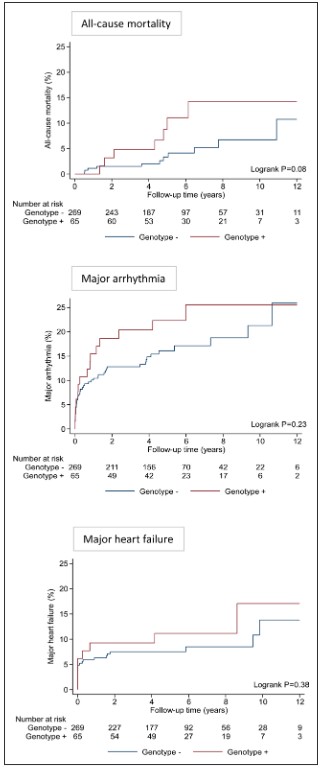
Over a median follow-up of 5.0 years (IQR, 3.9–7.8 years), there was a trend toward greater all-cause mortality in genotype-positive patients compared with genotype-negative patients (5-year mortality risk 11.1% vs 3.3%; P=0.08 after adjusting for age and sex).
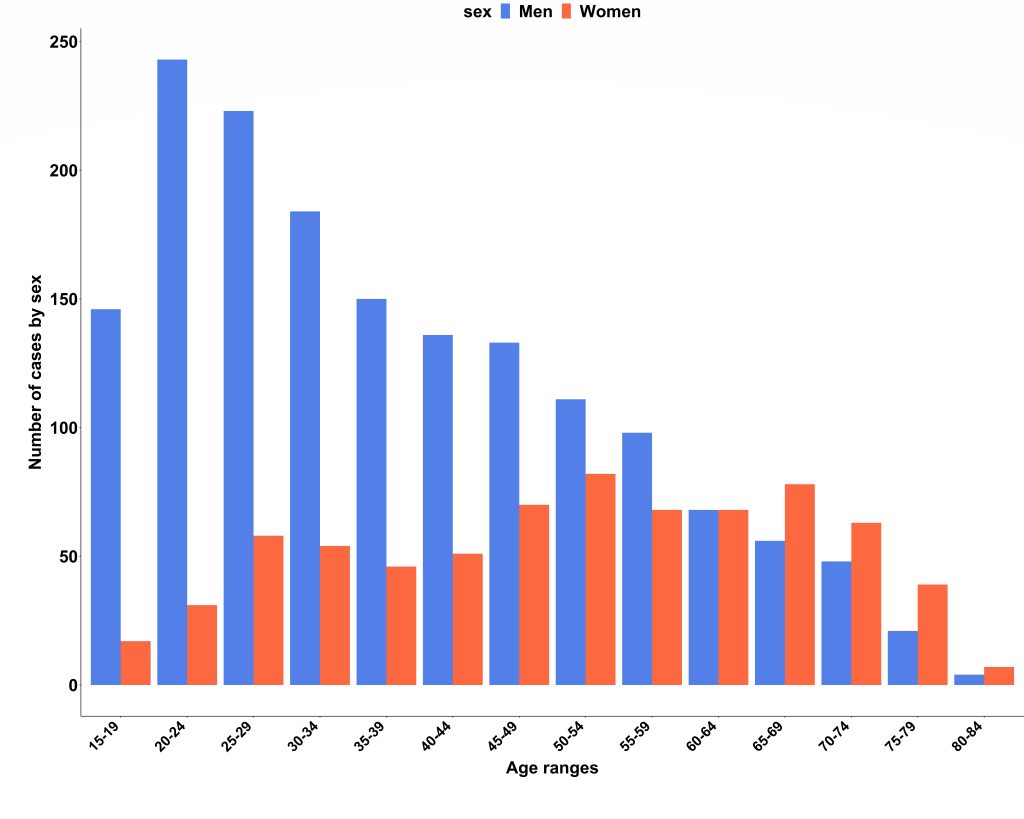
We obtained national hospital admission data from NHS Digital (3) and found there were 2353 admissions due to acute myocarditis over the 2-year study period (69% men; median age, 40 years; IQR, 27–55 years). Case ascertainment was calculated at 66%, and the London cohort was confirmed to be representative of national myocarditis admissions. Interestingly, we also found that men were significantly younger than women on admission to hospital (median age 35 years vs 52 years; P<0.001).
In conclusion, in this population-based study ~1 in 13 patients presenting with acute myocarditis were found to have an underlying variant in a gene robustly linked to DCM or ACM that would be reported as likely pathogenic in a patient with cardiomyopathy, compared with <1% in healthy controls. The presence of these variants affected clinical outcomes, particularly with DCM variants being associated with a trend towards greater all-cause mortality.
This study provides novel insights into why a small but important subset of patients with myocarditis experience major adverse events, whilst the majority usually recover spontaneously. It supports the concept that genotype-positive individuals may remain phenotypically silent until the occurrence of an environmental trigger.
This study suggests that genetic counselling and testing should be considered in patients with acute myocarditis, particularly in those with greater LV dysfunction, arrhythmia, or family history of cardiomyopathy. This may inform risk stratification and clinical management, including the need for ongoing surveillance and family screening, when cardiomyopathy-associated genetic variants are present.
A team of researchers with expertise across various disciplines was needed to harness the latest advances in precision phenotyping and genotyping whilst also leveraging national epidemiological datasets to deliver this study. Further work is underway to replicate these findings in a larger multi-centre setting and to provide greater mechanistic insights into genetic predisposition and disease progression in inflammatory cardiomyopathy using a systems biology approach.
The study was published under open access in Circulation on 26th Sept 2022:
The study was also selected for coverage on the American Heart Association News:
Genetics may explain rare heart inflammation in some young people | American Heart Association
Read the news story at the MRC London Institute of Medical Sciences
Genetics may explain rare heart inflammation in young people and athletes | MRC LMS
References:
- Grun S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert EM, Hill S, Ong P, Klingel K, et al. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. Journal of the American College of Cardiology. 2012;59:1604-1615
- Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. European heart journal. 2013;34:2636-2648, 2648a-2648d.
- https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics